Biosimilars vs Generics: Key Differences Explained

When you hear biosimilars and generics, you might think they’re the same thing-cheaper versions of brand-name drugs. But they’re not. Not even close. One is like copying a simple recipe. The other is trying to rebuild a jet engine using the same blueprints, but without knowing how the original was built. If you’re paying for a biologic drug like Humira or Enbrel, understanding this difference could save you thousands.
What Are Generics, Really?
Generics are the old reliable workhorses of the drug world. They’re small-molecule pills made from chemicals you can mix in a lab. Think ibuprofen, metformin, or lisinopril. Once the patent on the brand-name version expires, any company can make it-exactly the same chemical structure, same dose, same effect. The FDA doesn’t need to run new clinical trials. They just check that the generic absorbs into your bloodstream at the same rate and amount as the original. That’s called bioequivalence.That’s why generics cost 40% to 50% less than brand drugs. They’re cheap to make. A typical generic drug development costs between $2 million and $5 million. And because they’re chemically identical, pharmacists can swap them in automatically without asking your doctor. All 50 states allow this. No paperwork. No hesitation.
Over 90% of all prescriptions filled in the U.S. are generics. That’s 6.8 billion prescriptions a year. But they only make up about 20% of total drug spending because they’re so inexpensive. If you’ve ever picked up a $4 prescription at Walmart, you’re holding a generic.
What Are Biosimilars?
Biosimilars are different. They’re not copies. They’re highly similar versions of complex biologic drugs. Biologics aren’t made in a beaker. They’re grown in living cells-like yeast or hamster ovary cells. These drugs are huge proteins, sometimes thousands of times bigger than a generic drug molecule. Examples include Humira (adalimumab), Enbrel (etanercept), and Herceptin (trastuzumab). They treat conditions like rheumatoid arthritis, Crohn’s disease, and certain cancers.Because they’re made from living cells, no two batches are exactly alike. Even the original manufacturer can’t make them perfectly identical from one batch to the next. So when a biosimilar is made, it doesn’t have to be identical-it just has to be so close that it works the same way in your body, with no extra risk.
That’s why developing a biosimilar costs $100 million to $200 million. It’s not just about chemistry. It’s about proving similarity through hundreds of lab tests, animal studies, and sometimes clinical trials. The FDA requires over 200 analytical tests to compare structure, function, and purity. And unlike generics, biosimilar makers can’t copy the original company’s manufacturing process. They have to reverse-engineer it.
Why You Can’t Just Swap Biosimilars Like Generics
Here’s where things get tricky. With generics, your pharmacist can swap them in without telling you or your doctor. With biosimilars? Not so fast.Only biosimilars labeled as “interchangeable” can be swapped automatically. And as of late 2023, only 7 out of the 42 FDA-approved biosimilars have that status. The rest? Your doctor has to specifically prescribe them. Why? Because biologics can trigger immune reactions. If your body starts reacting to one version of Humira, switching to another-even a biosimilar-could cause side effects. That’s not a risk with generics.
Think of it like switching from one brand of paint to another. If it’s just a different shade of white, it’s fine. But if it’s a custom-mixed color with special additives, you need to know exactly what you’re using. That’s why rheumatologists and oncologists often prefer to stick with the same product unless there’s a clear cost benefit.
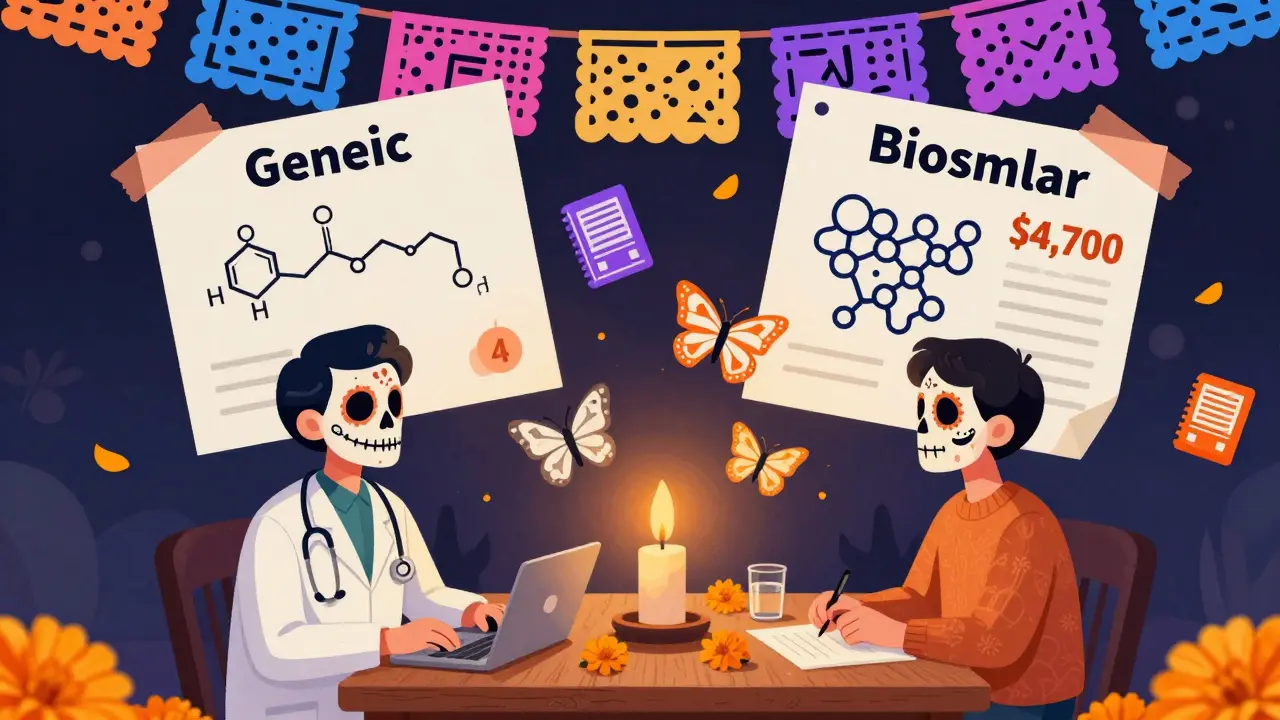
Cost Differences: It’s Not Just About the Price Tag
Generics save you 40-50%. Biosimilars? They save 15-20%-sometimes up to 33%. That sounds good, right? But here’s the catch: biologics are expensive to begin with. Humira costs about $7,000 a month without insurance. A 33% discount still leaves you paying $4,700. A generic for a blood pressure pill might drop from $100 to $4. That’s a real difference.And the savings don’t always reach patients. In the U.S., many biologics are sold under a “buy-and-bill” model. Doctors buy the drug and then bill insurance. If a biosimilar costs less, but the reimbursement rate doesn’t change, the doctor makes less profit. That creates a financial disincentive to switch. In Europe, where this model doesn’t exist, biosimilars make up 35% of the biologic market. In the U.S.? Less than 3%.
Regulatory Pathways: Two Different Systems
Generics are regulated under the Hatch-Waxman Act of 1984. Biosimilars? They follow the Biologics Price Competition and Innovation Act (BPCIA) of 2009. Two different laws. Two different standards.For generics: prove bioequivalence. For biosimilars: prove high similarity through analytical, nonclinical, and clinical data. The FDA doesn’t just look at blood levels. They check protein folding, sugar attachments, impurities, and how the drug interacts with immune cells.
The FDA keeps two public lists: the Orange Book for generics and the Purple Book for biologics and biosimilars. The Orange Book is simple: name, active ingredient, patent info. The Purple Book? It’s messy. It doesn’t list every detail because the science is too complex. That’s why patients and even some doctors struggle to understand what’s available.
Who’s Making These Drugs?
The generic market is crowded. Companies like Teva, Viatris, and Sun Pharma dominate. They make thousands of products. Biosimilars? It’s a tight club. Only a few players have the tech and money to play: Sandoz (Novartis), Samsung Bioepis, Amgen, and Pfizer. Each biosimilar takes years and hundreds of millions to develop. That’s why there are only 42 approved in the U.S. and why new ones are still rare.
What’s Changing in 2026?
The Inflation Reduction Act of 2022 is starting to have an effect. It caps insulin costs at $35 a month for Medicare patients and eliminates the Part D coverage gap. That’s pushing more people toward biosimilars. Also, major biologics like Stelara and Eylea are losing patents soon. That’s opening the door for dozens of new biosimilars by 2028.And the FDA is making it easier. New guidance allows extrapolation-approving a biosimilar for multiple uses based on data from just one condition. That’s speeding things up. Plus, the first interchangeable biosimilar for Humira, Amjevita, hit the market in January 2024 with a 35% discount. That’s a big deal.
But the biggest barrier isn’t science. It’s trust. Many doctors still worry about switching patients from a brand-name biologic to a biosimilar. A 2022 survey found 68% of rheumatologists wanted more training before prescribing biosimilars. That’s changing, slowly.
What This Means for You
If you’re on a generic drug, you’re probably fine. No need to worry. But if you’re on a biologic like Humira, Enbrel, or Remicade, ask your doctor: Is there a biosimilar available? Could I save money? Could I switch?Don’t assume your insurance will automatically switch you. Biosimilars often need prior authorization. Your pharmacy might not even stock them. Talk to your provider. Ask about the Purple Book. Check if your drug has an interchangeable version.
And remember: biosimilars aren’t generics. They’re not cheaper because they’re easier to make. They’re cheaper because they’re the next step in competition. But they’re not perfect replacements. They’re highly similar. And that’s enough-for most people, most of the time.
Are biosimilars the same as generics?
No. Generics are chemically identical copies of small-molecule drugs. Biosimilars are highly similar but not identical versions of complex biologic drugs made from living cells. They’re not interchangeable unless specifically approved as such by the FDA.
Can pharmacists substitute biosimilars without a doctor’s approval?
Only if the biosimilar has been designated as “interchangeable” by the FDA. As of 2026, only 7 out of 42 approved biosimilars have this status. For all others, your doctor must prescribe the specific product. Generics, by contrast, can be swapped automatically in all U.S. states.
Why are biosimilars more expensive to develop than generics?
Biosimilars are made from living cells, which makes their structure far more complex than small-molecule generics. Manufacturers must run hundreds of lab tests, animal studies, and clinical trials to prove similarity. Generic manufacturers only need to show bioequivalence through blood level tests. Biosimilar development costs $100-200 million; generics cost $2-5 million.
Do biosimilars work as well as the original biologic drugs?
Yes. The FDA requires that biosimilars have no clinically meaningful differences in safety, purity, or potency compared to the reference product. Thousands of patients have switched to biosimilars without issues. But because biologics can affect the immune system, switching should be done carefully under medical supervision.
How much do biosimilars save compared to brand-name biologics?
Biosimilars typically cost 15% to 33% less than the original biologic. For example, the first interchangeable Humira biosimilar, Amjevita, launched with a 35% discount. That’s less than the 40-50% savings seen with generics, but still significant given that biologics often cost over $7,000 a month.
Why aren’t more biosimilars available in the U.S.?
Several factors slow adoption: complex manufacturing, high development costs, patent thickets (like AbbVie’s 240+ patents on Humira), and reimbursement models that don’t incentivize providers to switch. Also, many doctors and patients are still unfamiliar with biosimilars. Europe has adopted them faster because of different pricing and substitution rules.
What’s Next?
The next five years will be critical. More biologics will lose patent protection. More biosimilars will come to market. And if the FDA continues to streamline approval and insurers start aligning reimbursement with cost savings, biosimilars could become the norm-not the exception.Right now, the biggest barrier isn’t science. It’s awareness. If you’re paying for a biologic, ask your doctor about biosimilars. Ask your pharmacist if there’s a cheaper option. And don’t assume your insurance will do the work for you. Knowledge is your best tool to cut costs without cutting corners on care.






So generics are like swapping out a lightbulb, and biosimilars are like trying to rebuild the whole electrical grid using the same blueprint but no wiring diagram? That’s wild.
Man, I love how this breaks it down-generics? Simple. Biosimilars? Like trying to clone a symphony orchestra… but one violinist is always slightly off-key, and the conductor’s mood changes every Tuesday. The FDA’s got a whole new dictionary for this stuff. Purple Book? More like a haunted encyclopedia.
And don’t get me started on the reimbursement mess-doctors get paid the same whether they give you the $7k drug or the $4.5k biosimilar? That’s like paying a chef the same wage whether they serve you steak or a really good veggie burger. Why would they switch?!
It’s not about science-it’s about incentives. And trust. And fear. And the fact that no one’s ever sat down with a patient and said, “Hey, this new version might work better… or it might make you break out in hives. But hey, it’s cheaper!”
Also, why does the Purple Book look like it was typed by a grad student on three espressos? Who designed that interface?!
I’ve seen patients cry because they couldn’t afford Humira. Then I’ve seen them panic because their pharmacist tried to swap them to a biosimilar without telling them. We need better education. Not just for docs. For everyone.
And yes, I’m screaming into the void. Again. But someone has to.
so biosimilars are not generics? got it. thanks for explain
Why does America even let foreign companies make these? India and Europe are already dominating biosimilar production. We’re stuck with patent trolls and overpriced drugs because our pharma CEOs care more about stock prices than patients. Time to nationalize biologics.
This is one of the clearest explanations I’ve ever read. I’ve been on a biologic for years and never understood why my insurance wouldn’t switch me. Now it makes sense. Thanks for writing this.
OMG YES!! I just switched to a biosimilar last month and my doc didn’t even tell me it wasn’t the original!! I thought it was a generic!! I was like ‘wait, why is my rash acting up??’ Turns out I had a mild immune reaction!!
PLEASE, doctors, just TELL us when you switch!! We’re not dumb-we just don’t know the difference between Purple Book and Orange Book!!
Also, why is the Purple Book so confusing?? Like, who thought ‘let’s make a drug database look like a 2007 Excel spreadsheet’ was a good idea??
Also also-can we PLEASE make biosimilars interchangeable already?? I’m tired of paperwork.
Structural homology, post-translational modifications, immunogenicity profiles-all critical differentiators. Bioequivalence ≠ biosimilarity. The regulatory burden reflects biological complexity.
Don’t let anyone tell you biosimilars aren’t worth it. I’ve been on one for a year. My joint pain? Gone. My bank account? Still breathing. This isn’t just science-it’s survival. If you’re scared to switch, talk to your doc. But don’t let fear cost you your health.
Why are we even talking about this? The U.S. spends more on drugs than every other country combined. Fix the system. Don’t just swap pills.
To everyone asking if biosimilars are safe: I’m a nurse. I’ve seen patients switch. Most do fine. A few have reactions. But here’s the thing-we don’t let people die because they’re afraid to try something new. We educate. We monitor. We support. This isn’t a risk-it’s progress. And we owe it to patients to stop treating them like lab rats.