The Connection between Allopurinol and Metabolic Syndrome
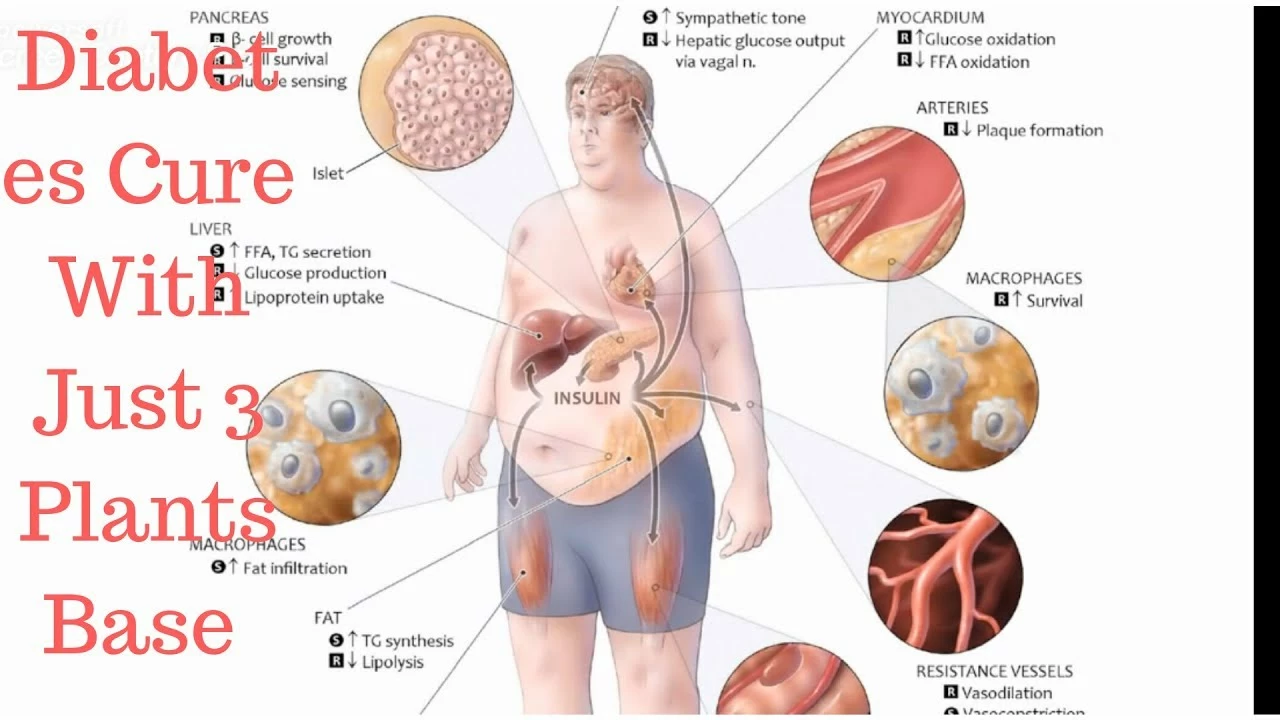
Understanding Allopurinol and Its Impact on Metabolic Syndrome
Many of us might not be familiar with the term "Allopurinol" and its connection to metabolic syndrome. Allopurinol is a medication commonly used to treat gout, a form of arthritis caused by the buildup of uric acid crystals in the joints. On the other hand, metabolic syndrome is a cluster of conditions that occur together, increasing your risk of heart disease, stroke, and type 2 diabetes. In this section, we will explore the basics of allopurinol, its primary use, and how it relates to metabolic syndrome.
Allopurinol belongs to a class of drugs called xanthine oxidase inhibitors, which work by reducing the production of uric acid in the body. High levels of uric acid can lead to gout, kidney stones, and even kidney failure. Metabolic syndrome, as mentioned earlier, consists of a group of risk factors that can lead to severe health issues. These risk factors include abdominal obesity, high blood pressure, high blood sugar levels, high triglycerides, and low HDL (good) cholesterol levels.
Now that we have a basic understanding of both allopurinol and metabolic syndrome, let's dive deeper into their connection and the potential benefits allopurinol might have on individuals with metabolic syndrome.
Allopurinol's Role in Reducing Inflammation and Oxidative Stress
Inflammation and oxidative stress play significant roles in the development and progression of metabolic syndrome. Allopurinol has been shown to possess anti-inflammatory and antioxidant properties, which could potentially be beneficial for individuals with metabolic syndrome. Research has demonstrated that allopurinol can reduce inflammation by inhibiting the production of pro-inflammatory cytokines and decreasing oxidative stress by scavenging free radicals.
These anti-inflammatory and antioxidant effects of allopurinol could help mitigate some of the complications associated with metabolic syndrome, such as insulin resistance, endothelial dysfunction, and cardiovascular disease. Therefore, it is essential to further explore the potential benefits of allopurinol for individuals with metabolic syndrome and the possible mechanisms by which it can improve their health.
Allopurinol's Impact on Insulin Resistance and Blood Sugar Levels
Insulin resistance is a significant factor in the development of metabolic syndrome and can lead to type 2 diabetes if left untreated. Studies have shown that allopurinol may help improve insulin sensitivity and reduce blood sugar levels in individuals with insulin resistance, thus potentially playing a role in the management of metabolic syndrome.
By decreasing inflammation and oxidative stress, allopurinol may help improve the function of insulin-producing cells in the pancreas and increase insulin sensitivity in peripheral tissues. This could lead to better blood sugar control and a reduced risk of developing type 2 diabetes in individuals with metabolic syndrome. Further research is needed to confirm these findings and establish the optimal dose and duration of allopurinol treatment for individuals with insulin resistance and metabolic syndrome.
Allopurinol's Effect on Blood Pressure and Cardiovascular Health
High blood pressure is another critical component of metabolic syndrome and a significant risk factor for cardiovascular disease. Allopurinol has been shown to have a positive impact on blood pressure regulation and overall cardiovascular health. By reducing inflammation and oxidative stress, allopurinol may help improve endothelial function, which is essential for maintaining healthy blood pressure levels.
Additionally, allopurinol has been shown to lower blood pressure in some individuals with hypertension, further supporting its potential role in managing metabolic syndrome. However, more studies are needed to establish the optimal dose, duration, and long-term effects of allopurinol on blood pressure and cardiovascular health in individuals with metabolic syndrome.
Conclusion: The Potential of Allopurinol in Metabolic Syndrome Management
Allopurinol, primarily known for its role in treating gout, has shown potential in managing various aspects of metabolic syndrome. Its anti-inflammatory and antioxidant properties may help reduce inflammation and oxidative stress, which are significant contributors to metabolic syndrome development and progression. Furthermore, allopurinol may help improve insulin resistance, blood sugar control, blood pressure regulation, and overall cardiovascular health in individuals with metabolic syndrome.
While these findings are promising, more research is needed to determine the optimal dose, duration, and long-term effects of allopurinol in individuals with metabolic syndrome. It is essential to consult with your healthcare provider before starting any new medication, including allopurinol, to ensure it is safe and appropriate for your specific situation.







Allopurinol, traditionally employed for gout management, presents a fascinating intersection with metabolic syndrome that warrants thorough examination. The pharmacological inhibition of xanthine oxidase reduces uric acid synthesis, thereby mitigating the crystalline deposition that precipitates gout attacks. Beyond its primary indication, emerging evidence suggests that this reduction in uric acid may attenuate oxidative stress pathways implicated in insulin resistance. By curbing the overproduction of reactive oxygen species, allopurinol may preserve endothelial function, a critical factor in maintaining vascular health. Moreover, inflammatory cytokine cascades, particularly those involving interleukin-6 and tumor necrosis factor-alpha, appear to be dampened under allopurinol therapy. This anti‑inflammatory effect holds promise for individuals burdened by the chronic low‑grade inflammation characteristic of metabolic syndrome. Clinical observations also hint at modest improvements in fasting glucose levels, although the magnitude of this effect remains contested. Blood pressure modulation represents another avenue of interest, as some trials have reported modest reductions in systolic readings among hypertensive cohorts receiving allopurinol. It is essential, however, to recognize the heterogeneity of patient responses, which may be influenced by genetic polymorphisms affecting drug metabolism. Safety considerations cannot be overlooked; while allopurinol is generally well‑tolerated, rare hypersensitivity reactions demand vigilance. The therapeutic window for optimal cardiovascular benefit remains undefined, necessitating rigorously designed dose‑finding studies. In parallel, the interplay between gut microbiota alterations and allopurinol’s metabolic impact deserves further investigation, given the microbiome’s role in glucose homeostasis. From a cost‑effectiveness standpoint, repurposing an established drug may offer a pragmatic solution for resource‑constrained healthcare systems. Nonetheless, clinicians must balance optimism with a cautious appraisal of the current evidence base. Future randomized controlled trials with robust endpoints will be pivotal in delineating allopurinol’s place within the armamentarium against metabolic syndrome. Until such data emerge, shared decision‑making with patients, emphasizing potential benefits and risks, remains paramount. In summary, the confluence of anti‑oxidative, anti‑inflammatory, and metabolic effects positions allopurinol as a candidate worthy of deeper scientific scrutiny.
While the enthusiasm for repurposing allopurinol is commendable, one must question the moral responsibility of prescribing a medication with a primary indication unrelated to metabolic health. It is ethically dubious to promote off‑label use without unequivocal, large‑scale evidence, especially when potential side effects, however rare, could jeopardize patient safety. Advocating widespread adoption based on preliminary findings borders on irresponsible, and the medical community should uphold rigorous standards before endorsing such practices.
Yo, imagine a world where a gout pill becomes the secret superhero fighting the fat‑fire of metabolic syndrome! It's like swapping your boring accountant for a rockstar poet who also happens to knock out uric acid. The drama of inflammation meets the philosophy of insulin resistance, and allopurinol steps onto the stage, wielding antioxidant vibes like a lyrical sword. Who knew chemistry could be this poetic?
i think its cool that a gout med could help with uric acid and sugar level its like two birds with one stone
this could be good for ppl who have both gout and metablic issues i hope doctors consider it
One might argue that applauding allopurinol's peripheral benefits is a manifestation of intellectual elitism, an attempt to cast a mundane pharmaceutical into the realm of avant‑garde therapeutics. Yet, such contrarian enthusiasm often disregards the pragmatic constraints of evidence‑based medicine, substituting conjecture for rigor.
Behold! The alchemy of allopurinol transforms from a humble gout guardian into a flamboyant champion of metabolic balance, wielding crimson fire against the icy grasp of insulin resistance! Its luminous aura dances across vascular corridors, dazzling the very essence of oxidative chaos.
In contemplating the mechanistic intricacies of allopurinol's impact upon metabolic derangements, one must adopt a rigorously analytical framework. The drug's capacity to attenuate reactive oxygen species warrants a systematic exploration of downstream effects on insulin signaling pathways, endothelial function, and systemic hemodynamics. A methodical appraisal of randomized controlled trial data, juxtaposed with mechanistic in‑vitro findings, will elucidate the veracity of posited therapeutic benefits.
this is an interesting perspective on allopurinol and metabolic health it seems there could be a benefit but more data is needed
Let us correct the record: the term is "xanthine oxidase inhibitor," not "xanthine oxidase inhibiter." Moreover, attributing cardiovascular benefits to allopurinol without robust meta‑analysis is scientifically untenable. While patriotism for domestic pharmaceuticals is admirable, it must not eclipse rigorous appraisal of pharmacodynamics and epidemiological outcomes.
One must confront the dramatic irony that a drug designed to quell gout may yet serve as a bulwark against the insidious tide of metabolic syndrome. Yet, let us not be swayed by romanticized narratives; the empirical evidence must stand as our sovereign judge.
Seriously, the hype around allopurinol as a magic bullet for metabolic syndrome is just another overblown claim. Sure, it might have some peripheral effects, but we're not talking about a miracle cure here.
Wow, because nothing says "effective treatment" like a gout pill that barely nudges blood pressure. Guess we should all just pop a couple of those and call it a day.
Interesting.
It is important to recognize that diverse populations may experience varying outcomes with allopurinol, and inclusive research practices can help ensure equitable access to potential benefits.
Look, folks, allopurinol might lower blood pressure-yeah, maybe-but let’s not pretend it’s a cure‑all for every metabolic woe, okay?; research still needs to back it up.
It is patritic to push a foreign drug when we hav domestic solutions that are more cost‑efficent. Allopurinol might have some use but it shouldn't replace proven local treatments for metabolic issues.
The philosophical implications of repurposing a drug extend beyond pharmacology; they challenge our conceptual boundaries between disease domains, urging a re‑examination of therapeutic ontology.
If you're considering allopurinol for metabolic concerns, consult your physician to evaluate suitability, monitor parameters, and integrate it responsibly into a comprehensive management plan.
The evidence is still evolving, so staying informed about new research will help make balanced decisions regarding allopurinol's role in metabolic health.